Theme: Circular Safe Hospitals / Call: Urban Rural Circularity May 2023
Towards circular human and veterinary hospitals: mapping opportunities to reduce harmful pharmaceutical discharge
Researchers from Wageningen University, Wageningen Food Safety Research, Utrecht University and the University Medical Center Utrecht have joined forces to map medicine discharge in human and veterinary hospitals and to identify and develop intervention strategies.
This project yields the initial steps towards realizing zero discharge of pharmaceuticals from hospitals to the environment. An important step is the identification of the most relevant substances used in human and veterinary hospitals, based on known use and analytical detection in selected waste streams of high interest from an environmental protection and human health risk perspective. This includes the distribution within the hospital waste system of both unused medication and medication that is excreted after treatment, that enter various waste streams. Opportunities for mitigation measures for ‘hotspot’ waste streams are being explored.
This project will provide the basis for developing a strategy using both technology and management strategies aimed at reducing pharmaceutical contaminants entering the circular water and food system.
Project Aim
In a previous project conducted in 2022 (also funded by the i4CS seed fund) the project team identified priority pharmaceuticals and waste streams within the hospital using information from the human and veterinary hospital pharmacies. A mapping study was carried out to understand where the priority pharmaceuticals enter the water or other waste streams. Finally, it was investigated what monitoring and intervention strategies were already in place. Based on these results, the goal of the current project is to verify the mapping by analysis of these waste streams, to conduct a risk assessment of the veterinary hospital to further support development of mitigation strategies, and the actual testing of mitigation strategies for the human hospital. The project uses a tiered approach as presented in the figure.
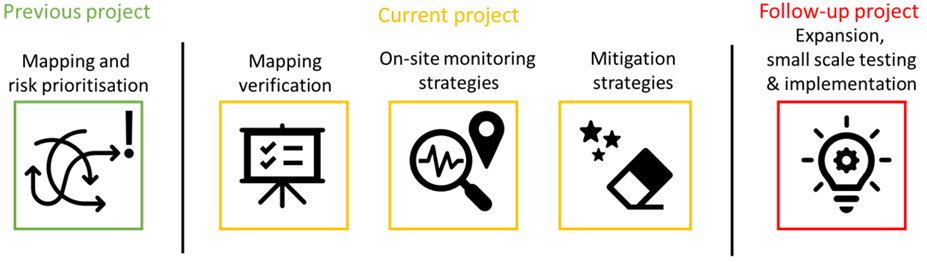
First step
The initial step involves verifying previous mapping results for the veterinary and human hospital by analyzing the most relevant hospital waste (water) streams identified and determining their convergence into larger and final collection points. During this process, the priority pharmaceuticals in the waste streams and their flux to the environment are quantified. Instrumental analysis of pharmaceuticals is labor-intensive and expensive, yet necessary for developing effective and focused mitigation strategies. Preselection of target pharmaceuticals (done in the previous project), along with analytical method development, optimization, and verification, is needed to achieve the most accurate data on pharmaceutical discharge. This accurate data is crucial for further investigating the development of successful intervention strategies and monitoring them effectively.
Second step
A second aim is the further development of a methodology to assess which residues in the veterinary hospital waste stream could be ‘high’ risk to human, environmental and ecological health by performing a risk assessment. This will follow-up on the preliminary work performed with the seed-money as well as the data produced in the analyses of pharmaceuticals in the waste streams.
Third step
The third part of this research will focus on identifying technologies that can be implemented in a decentralized way to treat the specific waste streams with high residue loads at their source in the hospitals. This study will focus of ICU urine bag waste streams. This will include investigating which technologies (filtration, absorption and oxidation) fit best with the identified pharmaceuticals in multiple compartments and waste streams. And which technologies are best suited for a decentralized and robust use on site. Potential technologies and the most effective mitigation strategies are identified based on an in depth literature review. This is followed by experimental exploration of possible technologies to be used for ICU urine bag waste streams.
Collaboration of main importance
The central focus of this project lies in fostering extensive collaboration and cross-pollination between human and veterinary hospitals. Both sectors encounter similar challenges in minimizing micropollutants. While some research has been conducted in human hospitals, it lacks a comprehensive approach, and no comparable studies have been carried out in veterinary hospitals. The incorporation of veterinary hospitals is crucial, given that their waste streams comprise not only wastewater but also manure, which serves as fertilizer in crop production. Consequently, pharmaceutical residues can find their way into water, feed, and food.
Follow-up
A final part of this project is searching for additional funding to further and more extensively study all relevant pharmaceuticals and waste streams. This follow-up project should yield the first small scale implementation of new mitigation strategies to achieve discharge with no environmental impact.
Results
Waste water sampling at the human hospital has been carried out over two weeks (two Wednesdays and two Sundays at two daily times each) at a total of four sampling points. The sampling points were selected in coordination with hospital staff to represent the overall hospital waste water discharge, the ICU related discharge, as well as the discharge from a normal ward. The detection and quantification of pharmaceuticals is currently being finalized and comprises a list of 15 target analytes, which will be used to validate pharmaceutical mass flux estimates that were created based on inventories as described above.

ICU Urine bag samples will be analyzed starting in the first week of March 2024. A literature based comparisons of potentially suitable on-site technologies is currently being finalized. Based on usability considerations as well as the technologies robustness and the avoidance of chemical use, sorption based mitigation measures were selected as good candidates for a more in depth investigation. We are exploring a range of different sorbent materials and combinations thereof and will experimentally test them on ICU Urine samples starting in March 2024.
Horse manure samples have been taken at the veterinary hospital at three different sampling days in 2023 during spring, summer and autumn to take into account any seasonal effects. In December 2023 all samples were analysed for 23 targeted analytes (e.g. antibiotics, painkillers, antiparasitic), resulting in the detection of the majority of the compounds, at low concentrations.

Publications
In October 2024 the team published their final report on this seed fund project. Please download it here.
Project Team
- Dr. Nora Sutton, Associate Professor Environmental Technology – Environmental Technology, Wageningen University and Research
- Dr. Gabriel Sigmund, Assistant Professor Environmental Technology – Environmental Technology, Wageningen University and Research
- Bochi Yu, Visiting Researcher – Environmental Technology, Wageningen University and Research
- Sifra van der Vis, MSc, Program Leader Statutory Tasks Veterinary Drugs -Wageningen Food Safety Research, Wageningen University and Research
- Dr. Serena Rizzo, Researcher Veterinary drugs – Wageningen Food Safety Research, Wageningen University and Research
- Prof. Dr. Ronette Gehring, DVM, Professor of Veterinary Pharmacotherapeutics and Pharmacy – Faculty of Veterinary Medicine, Utrecht University
- Dr. Huifang Deng, postdoctoral researcher – Institute for Risk Assessment Sciences, Faculty of Veterinary Medicine, Utrecht University
- Dr. Inge van Geijlswijk, Hospital pharmacist – clinical pharmacologist, Director of Pharmacy – Faculty of Veterinary Medicine, Utrecht University
- Sietske J. Mesu, PharmD – Faculty of Veterinary Medicine, Utrecht University
Contact
Assistant Professor Environmental Technology (WUR)
gabriel.sigmund@wur.nl
Program Leader Statutory Tasks Veterinary Drugs (WUR)
sifra.vandervis@wur.nl